COVID-19 has affected everyone in the world in one way, shape or form. With its outbreak and devastating effects, health care professionals have been tasked with developing methods of detection, treatment, and prevention. The U.S. Food and Drug Administration (FDA) has authorized the use of convalescent plasma as an Emergency Use Authorization (EUA) during this pandemic since there are limited treatment options for COVID-19. The process of using plasma to help aid in the treatment of diseases has been used for over a century, dating back to the 1918 influenza pandemic and more recent the 2003 outbreak of severe acute respiratory syndrome (SARS).
 So what exactly is convalescent plasma? One of the ways that our bodies fight off infectious diseases is by developing antibodies that lead to the destruction of the invading microorganism. These antibodies are present in the blood, more specifically the liquid portion of the blood called plasma. Convalescent plasma is plasma that has been donated from a person who has been recently infected with a disease and has since recovered from it. People who have been infected with COVID-19 have antibodies to the virus. Their donated plasma (now containing the antibodies) can be given to individuals who are ill with COVID-19 in an effort to help them fight the virus and get better.
So what exactly is convalescent plasma? One of the ways that our bodies fight off infectious diseases is by developing antibodies that lead to the destruction of the invading microorganism. These antibodies are present in the blood, more specifically the liquid portion of the blood called plasma. Convalescent plasma is plasma that has been donated from a person who has been recently infected with a disease and has since recovered from it. People who have been infected with COVID-19 have antibodies to the virus. Their donated plasma (now containing the antibodies) can be given to individuals who are ill with COVID-19 in an effort to help them fight the virus and get better.
Here at the Johns Hopkins Hospital Blood Bank, we store and dispense a number of convalescent plasma units. Our inventory is provided by collection centers including the American Red Cross, the New York Blood Center, and the Blood Bank of Delmarva. Once donated, plasma is good for one year. We store our plasma in freezers kept at ≤-18°C. When requested, the plasma is thawed in a 37°C water bath and must be transfused within 5 days.
Johns Hopkins researchers have received funding from the U.S. Department of Defense for two nationwide clinical trials to test the effectiveness of a convalescent plasma outpatient treatment. The trials are being conducted at over 20 ambulatory clinics in medical centers across the U.S. Researchers hope to determine whether convalescent plasma transfusions can effectively be used to treat people in the early stages of COVID-19 illness or prevent the infection in those at high risk of exposure to the virus at their home or jobs.
Managing these studies is an added task to the daily workflow of the Blood Bank technologists. Study participants are randomized to receive either convalescent (plasma containing antibodies to the COVID-19 virus) or control plasma (plasma lacking antibodies to the COVID-19 virus). Many of the infusions occur off of the Johns Hopkins Medical Campus, therefore the plasma has to be packed in a container and transported to the transfusion site. Blood Bank technologists work effortlessly to prepare the study plasma for transport while routine and/or trauma transfusions continue.
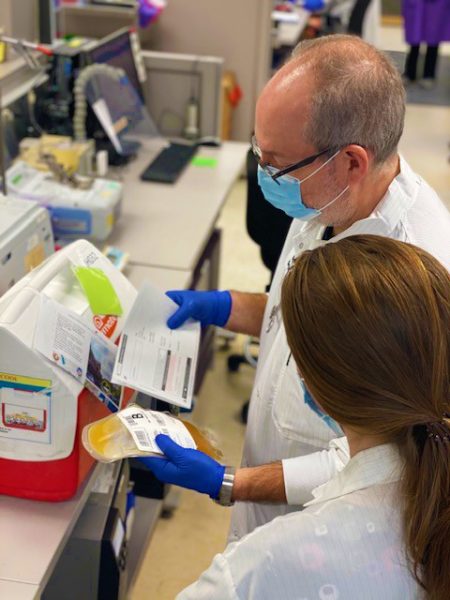
Each unit is thawed, relabeled and blinded in order to prevent the study team from knowing which type of plasma the participant will receive. After attaching a product requisition and performing a verbal verification with another technologist, the plasma is placed into a cooler on top of a bag of ice and delivered via courier to the clinic. Convalescent plasma is also used to treat inpatients at the hospital with COVID-19 infections. Between April 1, 2020 and December 31, 2020 there have been 282 units of convalescent plasma dispensed from the Johns Hopkins Hospital Blood Bank, with 265 patients (study and inpatient) having received at least one unit. More information on the COVID-19 plasma study can be found at covidplasmatrial.org. If you are interested in donating any type of blood product, please contact your local American Red Cross.
Rivcah Davis, MS, BB(ASCP)CM
Transfusion Coordinator II – Transfusion Medicine Division